How to Choose the Lab pH Meters
Goodmorning! I’m Eric Chen from best2buy.reviews. I’m very happy to stay here and share you some information as well as experirence to choose Lab pH Meters. Hope that it’s useful to choose right products! Let’s explore it now!z`
- 1. What are Lab pH Meters?
- 2. What does Lab pH Meters consist of?
- 3. How does Lab pH Meters work?
- 3.1. Calibration:
- 3.2. Measurement:
- 3.3. Maintenance:
- 4. Types of Lab pH Meters
- 5. Applications of Lab pH Meters
- 5.1. Chemistry Laboratories:
- 5.2. Biology and Biochemistry:
- 5.3. Environmental Monitoring:
- 5.4. Water Treatment Plants:
- 5.5. Food and Beverage Industry:
- 5.6. Pharmaceutical Manufacturing:
- 5.7. Agriculture:
- 5.8. Medical Research and Clinical Laboratories:
- 5.9. Diagnostics:
- 5.10. Education:
- 5.11. Industrial Processes:
- 5.12. Swimming Pool and Spa Maintenance:
- 6. How to choose Lab pH Meters?
- 6.1. Accuracy and Precision:
- 6.2. Resolution:
- 6.3. Measurement Range:
- 6.4. Temperature Compensation:
- 6.5. Calibration:
- 6.6. Ease of Use:
- 6.7. Type of Electrode:
- 6.8. Sample Volume and Sample Type:
- 6.9. Portability:
- 6.10. Multiparameter Capability:
- 6.11. Durability and Build Quality:
- 6.12. Brand and Supplier Reputation:
- 6.13. Budget:
- 7. In conclusion
What are Lab pH Meters?
Lab pH meters are instruments used to measure the acidity or alkalinity of a solution in a laboratory setting. pH is a measure of the concentration of hydrogen ions in a solution, and it is expressed on a scale ranging from 0 to 14. A pH of 7 is considered neutral, while values below 7 indicate acidity and values above 7 indicate alkalinity.
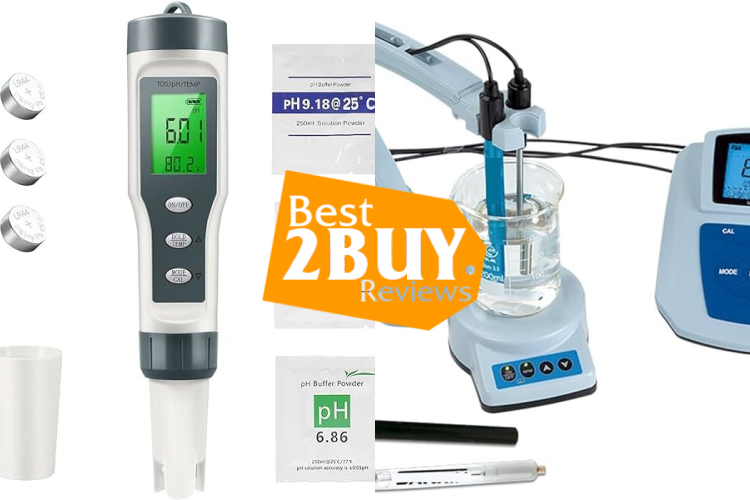
What does Lab pH Meters consist of?
Lab pH meters typically consist of a probe or electrode, a meter, and a reference electrode. The pH electrode is a special sensor that responds to the concentration of hydrogen ions in the solution. The reference electrode is used to provide a stable reference potential against which the pH electrode's signal can be measured. The meter displays the pH value based on the signals from the electrodes.
How does Lab pH Meters work?
Here's a brief overview of how lab pH meters work:
Calibration:
- Before use, pH meters need to be calibrated using buffer solutions with known pH values. This ensures the accuracy of the measurements.
Measurement:
- The pH electrode is immersed in the solution being tested, and it generates a voltage proportional to the hydrogen ion concentration. This voltage is then converted into a pH reading by the meter.
Maintenance:
- Proper maintenance is crucial for accurate and reliable measurements. Regular cleaning, calibration, and storage in appropriate solutions are common practices.
Lab pH meters are essential in various scientific fields, including chemistry, biology, environmental science, and medicine. They are used to monitor and control the pH of solutions in experiments, quality control processes, and various industrial applications. The accuracy of pH measurements is important in many processes, as slight changes in pH can have significant effects on chemical reactions, biological processes, and product quality.
Types of Lab pH Meters
Some common types:
Benchtop pH Meters:
- Description: These are larger, more robust pH meters designed for use on laboratory benches or workstations.
- Applications: Suitable for a wide range of laboratory applications, including research, quality control, and education.
Portable pH Meters:
- Description: Compact and lightweight pH meters designed for on-the-go measurements.
- Applications: Ideal for fieldwork, environmental monitoring, and situations where mobility is important.
Pen-Style or Pocket pH Meters:
- Description: Small, handheld devices resembling a pen for convenient and quick pH measurements.
- Applications: Commonly used for quick pH checks in the field or in simple laboratory tasks.
Multiparameter Meters:
- Description: Meters capable of measuring multiple parameters in addition to pH, such as temperature, conductivity, and dissolved oxygen.
- Applications: Useful in applications where comprehensive water quality analysis is required.
Micro pH Meters:
- Description: pH meters designed for measuring small sample volumes, often in the microliter range.
- Applications: Commonly used in biological and biochemical research where small sample sizes are crucial.
Online or Continuous pH Monitoring Systems:
- Description: Systems that continuously monitor and record pH levels in real-time.
- Applications: Used in industrial processes, water treatment plants, and other applications where continuous monitoring is essential.
pH Indicator Strips:
- Description: Paper or plastic strips impregnated with pH-sensitive dyes that change color based on the pH of a solution.
- Applications: Quick and cost-effective for approximate pH measurements, often used in educational settings.
Laboratory pH Electrodes:
- Description: While not a meter itself, pH electrodes are a crucial component. They can be attached to a meter and are available in various types, such as glass combination electrodes, reference electrodes, and specialty electrodes for specific applications.
Applications of Lab pH Meters
Lab pH meters find applications across various scientific disciplines and industries due to the importance of pH in understanding and controlling chemical and biological processes. Here are some common applications:
Chemistry Laboratories:
- pH meters are extensively used in chemistry labs for determining the acidity or alkalinity of solutions. This is crucial in various chemical reactions, titrations, and synthesis processes.
Biology and Biochemistry:
- In biological and biochemical research, pH control is vital for maintaining the stability and functionality of biological molecules, enzymes, and cell cultures.
Environmental Monitoring:
- pH meters are used to assess the acidity or alkalinity of natural water bodies, soil, and air. Monitoring pH in environmental samples is essential for understanding ecosystem health and potential impacts on aquatic life.
Water Treatment Plants:
- pH is a critical parameter in water treatment processes. pH meters are employed to monitor and control the pH levels in drinking water, wastewater, and industrial water treatment plants.
Food and Beverage Industry:
- pH is a key factor in food and beverage production, affecting taste, texture, and shelf life. pH meters are used for quality control in food processing to ensure products meet desired specifications.
Pharmaceutical Manufacturing:
- pH control is crucial in pharmaceutical manufacturing processes to ensure the stability and efficacy of drugs. pH meters are used in formulation, quality control, and research and development.
Agriculture:
- Soil pH affects nutrient availability to plants. pH meters are used in agriculture to assess soil acidity or alkalinity, helping farmers make informed decisions about soil amendments and crop management.
Medical Research and Clinical Laboratories:
- In medical research and clinical laboratories, pH meters are used in various applications, including cell culture maintenance, diagnostic testing, and research involving biological fluids.
Diagnostics:
- pH measurements play a role in diagnostic tests, such as blood gas analysis, where the pH of blood is a critical parameter indicating the body's acid-base balance.
Education:
- pH meters are commonly used in educational settings to teach students about the principles of acidity and alkalinity, as well as the use of analytical instruments.
Industrial Processes:
- Many industrial processes require careful pH control to optimize chemical reactions and product quality. Industries such as textiles, pulp and paper, and chemical manufacturing utilize pH meters for process monitoring and control.
Swimming Pool and Spa Maintenance:
- pH is critical for maintaining the effectiveness of sanitizers in swimming pools and spas. pH meters are used to ensure proper water balance and prevent issues such as corrosion or microbial growth.
How to choose Lab pH Meters?
Some key considerations to help guide your decision:
Accuracy and Precision:
- Consider the required level of accuracy for your measurements. High-precision applications, such as research or quality control in sensitive processes, may require more advanced pH meters with greater accuracy.
Resolution:
- The resolution of a pH meter refers to the smallest pH unit it can display. Higher resolution is beneficial for applications where small pH changes are significant.
Measurement Range:
- Ensure that the pH meter's measurement range covers the expected pH values in your samples. Some applications may require a broader range, especially if you work with highly acidic or alkaline substances.
Temperature Compensation:
- pH readings can be influenced by temperature changes. Choose a pH meter with automatic temperature compensation (ATC) to account for temperature variations, especially if your samples are not at room temperature.
Calibration:
- Check the calibration process of the pH meter. Some meters allow for one-point calibration, while others may offer two or more points. Consider the ease of calibration and the availability of standard buffer solutions.
Ease of Use:
- Choose a pH meter that is user-friendly, especially if it will be used by multiple individuals. Features such as a clear display, intuitive controls, and straightforward calibration procedures can enhance usability.
Type of Electrode:
- Different applications may require specific types of pH electrodes. Common types include glass combination electrodes, reference electrodes, and specialty electrodes for specific applications. Ensure that the chosen electrode is suitable for your sample type.
Sample Volume and Sample Type:
- Consider the volume of your samples and the compatibility of the pH meter with your sample type. Some applications, such as microscale experiments or fieldwork, may require specific pH meter designs.
Portability:
- Determine whether you need a benchtop pH meter for a dedicated lab space or a portable model for fieldwork. Portable meters are often battery-powered and suitable for on-the-go measurements.
Multiparameter Capability:
- If you need to measure additional parameters alongside pH (e.g., temperature, conductivity), consider a multiparameter meter for efficiency and convenience.
Durability and Build Quality:
- Evaluate the durability and build quality of the pH meter. This is important, especially in industrial settings or fieldwork where the instrument may be subjected to harsh conditions.
Brand and Supplier Reputation:
- Consider the reputation of the brand and supplier. Reliable manufacturers often provide quality instruments and good customer support.
Budget:
- Determine your budget for a pH meter. While it's important to find a meter that meets your requirements, consider the cost-effectiveness and value for money.
Before making a final decision, it may be helpful to read user reviews, seek recommendations from colleagues, and consult with suppliers or manufacturers to ensure that the chosen pH meter aligns with your specific needs.
In conclusion
If you want to buy Lab pH Meters, check out websites. We noted top products which highly appreciated. You can refer and buy it in store or shopping online. If you buy online, check out Amazon by click: “Buy it on Amazon”, it’s very convenient. Hope you will find and satisfied with your selection.
Hope with our information, it’s useful for you to give decision. Kindly read carefully before buying anything. And don’t forget me! I’m Eric Chen from best2buy.reviews, I’m always available to help you.










